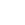モネイドケミカルズ
電話: 86-315-8309571
WhatsApp/WeChat/モバイル: 0086-15633399667
Skype: janet-honest
住所: 2-7-523 Jidong Building Materials Tangshan, Hebei 064000 China
|
化学名 |
ビシン |
|
CAS番号 |
|
|
分子式 |
C6H13NO4 |
|
EINECS番号 |
205-755-1 |
|
分子量 |
163.17 |
|
分子構造 |
|
|
詳細 |
Appearance: White crystalline Assay: 99.0%min. Water-solubility: colorless, transparent. Water: 0.5%max. pH: 4.3~5.3 |
|
主な用途 |
Used as slow granule in the biochemical studies. |
Bicine (N,N-bis(2-hydroxyethyl)glycine) is an organic zwitterionic buffer compound belonging to the Good's buffers family. This white crystalline powder exhibits excellent water solubility and maintains stable pH in biochemical systems. Its molecular structure contains both carboxylate and hydroxyl groups, allowing it to function effectively as an amphoteric buffer. Bicine is particularly valued for its minimal metal ion interference and stable pH range (7.6-9.0), making it suitable for sensitive biological applications. The compound's low ultraviolet absorbance and minimal enzyme inhibition contribute to its widespread use in protein research and molecular biology techniques.
What is bicine used for?
Bicine serves as an effective buffer in various biochemical and molecular biology applications, particularly in protein electrophoresis and chromatography. It's commonly employed in plant cell culture media and enzyme assays where traditional buffers may interfere. The compound finds special utility in crystallography studies of metalloproteins due to its low metal-chelation properties. Bicine-based buffers are also used in nucleic acid hybridization procedures and as a component in some diagnostic kits. Its stable buffering capacity at slightly alkaline pH makes it valuable for maintaining physiological conditions during cell fractionation and organelle isolation procedures.
What are the benefits of bicine?
Bicine offers several advantages including exceptional buffering capacity in the pH 7.6-9.0 range with minimal ionic strength effects. It shows negligible interference with biochemical reactions and exhibits low UV absorbance, enabling spectrophotometric analysis. The buffer causes minimal metal ion chelation compared to alternatives like Tris, making it ideal for metalloprotein studies. Bicine demonstrates excellent temperature stability and maintains consistent pH across various experimental conditions. Its zwitterionic nature reduces membrane permeability, minimizing cellular stress in biological systems. These properties combine to make it a superior choice for sensitive protein work and long-duration experiments requiring pH stability.