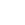Welcome to Tangshan Moneide Trading Co., Ltd.
Moneide Chemicals
Tel: 86-315-8309571
WhatsApp/WeChat/Mobile: 0086-15633399667
Skype: janet-honest
Mail: sales@moneidechem.com
Address: 2-7-523 Jidong Building Materials Tangshan, Hebei 064000 China
Eriochrome black T
|
Chemical Name |
Eriochrome black T |
|
Synonyms |
C.I. 14645; 3-Hydroxy-4-(1-hydroxy-2-naphthylazo)-7-nitro-1-naphthalene sulfonic acid sodium salt; Mordant Black 11 |
|
CAS No. |
1787-61-7 |
|
Molecular formula |
C20H12N3NaO7S |
|
EINECS No. |
217-250-3 |
|
Molecular weight |
461.38 |
|
Molecular Structure |
|
|
Details |
Appearance: brown black powder with metal luster Water Soluble Test: pass Solublity in Ethanol Passes Test: pass Sensitivity Test: pass Sulfated ash(as SO4): 20% max. |
|
Main Application |
Used as complexometric titration indicator. |
What is Eriochrome Black T used for?
Eriochrome Black T (EBT) is primarily used as a metallochromic indicator in complexometric titrations, especially for determining water hardness by measuring calcium and magnesium ions. This dye forms colored complexes with metal ions, enabling visual endpoint detection in EDTA titrations. Beyond analytical chemistry, it serves as a biological stain and finds applications in textile dyeing. The compound's ability to selectively bind certain metal ions makes it valuable for environmental monitoring of heavy metals in water systems. Its distinct color change properties also allow use in educational demonstrations of complexation chemistry.
Why is EBT used in EDTA titration?
EBT is ideal for EDTA titrations because it forms weaker complexes with Ca²⁺ and Mg²⁺ than EDTA does, ensuring the indicator releases these metals to EDTA during titration. The dye's sharp endpoint color change from wine-red to blue provides clear visual confirmation when all metal ions have been complexed by EDTA. Its selective reactivity with divalent cations prevents interference from other metals when properly buffered. The stability of its metal complexes allows for accurate determination of water hardness. These properties make EBT the preferred indicator for standard hardness titrations.
Is Eriochrome Black T indicator acidic or basic?
Eriochrome Black T is an amphoteric compound that functions as an acid-base indicator in addition to its metallochromic properties. In strongly acidic solutions (pH <6), it appears red, transitioning to blue in alkaline conditions (pH >11). However, for metal ion titrations, it's typically used in buffered alkaline solutions (pH 9-10) where the dye is blue and forms stable wine-red complexes with Ca²⁺ and Mg²⁺. This pH-dependent behavior requires careful buffer control during analytical applications to maintain proper indicator function.
What is the color change of Eriochrome black T?
Eriochrome Black T exhibits a characteristic color transition from blue to wine-red when complexing with calcium or magnesium ions in alkaline conditions. During EDTA titrations, the solution initially appears wine-red due to the metal-EBT complex. As EDTA is added, it sequesters the metal ions, causing the indicator to revert to its uncomplexed blue form at the titration endpoint. This vivid, easily distinguishable color change occurs sharply when all metal ions have been complexed by EDTA, providing reliable visual detection of the titration endpoint.