Produtos Químicos Moneide
Telefone: 86-315-8309571
WhatsApp/WeChat/Celular: 0086-15633399667
Skype: janet-honesta
Correspondência: sales@moneidechem.com
Endereço: 2-7-523 Jidong Building Materials Tangshan, Hebei 064000 China
|
Nome químico |
Bromothymol blue |
|
Nº CAS. |
76-59-5 |
|
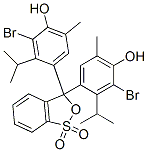
Fórmula molecular |
C27H28Br2O5S |
|
EINECS No. |
200-971-2 |
|
Peso molecular |
624.39 |
|
Estrutura Molecular |
|
|
Detalhes |
Appearance: Pale pinkish purple or red crystalline powder PH change range: 6.0(yellow)~7.6(blue) Solubility in alcohol: Passes test Sulfated ash: 0.3%max. Absorptivity(L/cm·g): 52min. Solubility: solve in alcohol, lye and hartshorn, not solve in water. Packing: 25kg/ fibre drum |
|
Aplicação principal |
Used as acid-base indicator. |
What is bromothymol blue, and what is it used for?
Bromothymol blue is a pH-sensitive synthetic dye that acts as a visual acid-base indicator, changing color between pH 6.0 (yellow) and 7.6 (blue). It is commonly used in laboratories to monitor pH shifts in chemical reactions, titrations, and microbiological tests. In biology, it helps detect carbon dioxide levels in respiration experiments, turning from blue to yellow as CO₂ increases acidity. It is also used in environmental testing and educational demonstrations due to its clear, reversible color transition.
What color will bromothymol blue turn in an acid?
In acidic conditions (pH below 6.0), bromothymol blue turns yellow. The dye's molecular structure shifts as it absorbs hydrogen ions, altering its light absorption properties. This color change makes it useful for identifying acidic environments in experiments, such as tracking fermentation or assessing water quality. At neutral pH (around 7.0), it appears green—a mix of its acidic (yellow) and basic (blue) forms.
Is bromothymol blue the same as methylene blue?
No, they are completely different compounds. Bromothymol blue is a pH indicator that changes color with acidity, while methylene blue is a redox dye used in medical treatments, staining, and chemistry. Their chemical structures and applications differ significantly—bromothymol blue detects pH shifts, whereas methylene blue stains cells, treats methemoglobinemia, and acts as an antioxidant.
Is bromothymol blue edible?
No, bromothymol blue is not safe to ingest. It is a laboratory chemical that can be harmful if swallowed, causing irritation or toxicity. While it is used in some educational and scientific settings, it is strictly for experimental purposes and should never be consumed. Food-grade pH indicators, like those in some candies or test strips, use safer alternatives for edible applications.