Moneide Chemicals
Tel: 86-315-8309571
WhatsApp/WeChat/Mobile: 0086-15633399667
Skype: janet-honest
Mail: sales@moneidechem.com
Address: 2-7-523 Jidong Building Materials Tangshan, Hebei 064000 China
Methylene blue trihydrate
|
Chemical Name |
Methylene blue trihydrate |
|
Synonyms |
Basic Blue 9 trihydrate; 3,7-Bis(dimethylamino)phenothiazin-5-ium chloride |
|
Molecular formula |
C16H18CLN3S·3H2O |
|
CAS No. |
7220-79-3 |
|
Molecular weight |
373.90 |
|
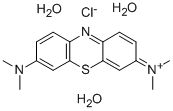
Molecular Structure |
|
|
Details |
Appearance: Bottle green crystal or crystalline powder with gunmetal shine, without smell. Assay: 98.0% min Change color range: Passes test Solubility in ethanol: Passes test Loss on drying: 15%max Sulfated ash: 0.5%max Biological stain: Passes test As: 0.0005%max Cu: 0.03%max Zn: 0.02%max Solubility: soluble in water, ethanol and chloroform, insoluble in aether, water solution is blue. Packing: 25kg/ fibre drum |
|
Main Application |
Used for REDOX indicator, adsorption indicator and biological dyeing agents. |
What is the use of methylene blue trihydrate?
Methylene blue trihydrate is a versatile compound primarily used in medical and biological applications. In medicine, it serves as an antidote for methemoglobinemia, converting abnormal methemoglobin back to functional hemoglobin. The trihydrate form is particularly valued in laboratory settings for histological staining and as a redox indicator in biochemical experiments. Aquaculture industries utilize it as an antifungal and antiparasitic treatment for fish. Its ability to stain living cells makes it valuable for microscopy, especially in nervous tissue visualization. The trihydrate formulation offers improved stability and solubility compared to anhydrous forms, making it preferable for precise pharmaceutical preparations and diagnostic procedures.
Is methylene blue trihydrate the same as methylene blue?
Methylene blue trihydrate and methylene blue share the same active compound but differ in their hydration state. The trihydrate form contains three water molecules (C₁₆H₁₈ClN₃S·3H₂O) per molecule of methylene blue, while the anhydrous version lacks these water molecules. This hydration affects physical properties like solubility and stability, with the trihydrate being more soluble in aqueous solutions. Pharmacologically, both forms have identical therapeutic effects as the water molecules dissociate in solution. However, the trihydrate is generally preferred for laboratory use due to its consistent performance in staining applications and better handling characteristics in powder form.
What is the difference between methyl blue and methylene blue?
Methyl blue and methylene blue are distinct chemical compounds with different structures and applications. Methyl blue (CI 42780) is a triarylmethane dye used primarily as a biological stain and pH indicator, while methylene blue (CI 52015) is a phenothiazine derivative with medical applications. Structurally, methyl blue contains multiple sulfonated aromatic rings, making it more water-soluble but less permeable through cell membranes. Methylene blue's smaller molecular size allows it to penetrate cells and participate in redox reactions, enabling its use in medical treatments. Their staining properties also differ significantly - methyl blue preferentially stains connective tissues, whereas methylene blue is used for nervous tissue and microbial staining.