Moneide Chemicals
Tel: 86-315-8309571
WhatsApp/WeChat/Mobile: 0086-15633399667
Skype: janet-honest
Mail: sales@moneidechem.com
Address: 2-7-523 Jidong Building Materials Tangshan, Hebei 064000 China
Tetrabutyl Ammonium Fluoride
|
Chemical Name |
Tetrabutyl ammonium fluoride |
|
Synonyms |
Tetrabutylammonium fluoride |
|
CAS No. |
87749-50-6 |
|
Molecular formula |
C16H36NF |
|
Molecular weight |
261.46 |
|
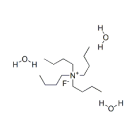
Molecular Structure |
|
|
Details |
Appearance: white to yellow waxy solid Purity: 82.0% min. |
|
Main Application |
The selectivity of fluorination catalyst and synthesis of chemical agent, silane protecting group removal agent. Such as halogen replacement reaction, redox reaction, alkylation of N-, CABBEEN two chlorine in the presence of phase transfer catalyst in organic synthesis. |
- Product Name: Tetrabutyl Ammonium Fluoride
- Synonyms: TBAF; Tetrabutylammonium fluoride solution; N,N,N - Tributyl - 1 - butanaminium fluoride
- Linear Formula: (C₄H₉)₄N(F)
- CAS Number: 429 - 41 - 4
- Molecular Weight: 261.45
- EC Number: 207 - 052 - 3
- MDL Number: MFCD00011824
- PubChem Substance ID: 24854531
- ຮູບລັກສະນະ: Usually appears as a colorless to pale yellow liquid in solution forms, or a white solid in anhydrous forms.
- Solubility: Soluble in common organic solvents such as THF (tetrahydrofuran), acetonitrile, and dichloromethane. In THF, it forms a stable and reactive solution, which is widely used in various chemical reactions.
- Deprotection Reactions: Tetrabutyl Ammonium Fluoride is widely used in deprotection reactions of silyl ethers. Silyl ethers are commonly used protecting groups in organic synthesis. TBAF selectively cleaves the silicon - oxygen bond, removing the silyl protecting group and regenerating the original hydroxyl or other functional groups. For example, in the synthesis of complex natural products, TBAF can be used to deprotect multiple silyl - protected functional groups in a controlled manner, enabling the construction of the desired molecular structure.
- Fluorination Reactions: It can also be employed in certain fluorination reactions. The fluoride ion provided by TBAF can react with suitable electrophiles to introduce fluorine atoms into organic molecules. This is particularly useful in the development of pharmaceuticals and agrochemicals, where the introduction of fluorine can significantly alter the biological activity and physicochemical properties of the molecules.
- Nanomaterial Synthesis: In the synthesis of nanomaterials, such as silica nanoparticles, Tetrabutyl Ammonium Fluoride can play a role in controlling the particle size and morphology. It can interact with silica precursors and influence the growth and aggregation of silica nanoparticles, leading to the formation of well - defined nanomaterials with specific properties.
- Polymer Modification: TBAF can be used to modify polymers. For instance, it can react with certain polymers containing reactive groups, introducing fluoride - containing moieties. This can enhance the polymer's properties, such as its chemical resistance, hydrophobicity, or electrical conductivity, depending on the nature of the polymer and the reaction conditions.
- As a Catalyst or Catalyst Promoter: In some chemical reactions, Tetrabutyl Ammonium Fluoride can act as a catalyst or a catalyst promoter. For example, in certain cycloaddition reactions, TBAF can facilitate the reaction by coordinating with reactants or by promoting the formation of reactive intermediates. Its ability to enhance reaction rates and selectivities makes it a valuable component in catalytic systems.
- Tetrabutyl Ammonium Fluoride is corrosive. It can cause severe skin burns and eye damage. Inhalation or ingestion of this compound can be harmful.
- The fluoride ion can also be toxic, and exposure to high concentrations can lead to adverse health effects, including damage to the respiratory system, digestive system, and bones.
- When handling Tetrabutyl Ammonium Fluoride, always wear appropriate personal protective equipment, including chemical - resistant gloves, safety goggles with side - shields, and a lab coat or protective apron.
- Work in a well - ventilated area, preferably under a fume hood, to avoid inhalation of vapors or dust.
- In case of contact with skin, immediately remove contaminated clothing and rinse the affected area with plenty of water for at least 15 minutes. Seek medical attention promptly. If the compound gets into the eyes, rinse the eyes with copious amounts of water for at least 15 minutes, lifting the eyelids occasionally, and then seek immediate medical help.
- Do not eat, drink, or smoke in the area where Tetrabutyl Ammonium Fluoride is being used.
- Store Tetrabutyl Ammonium Fluoride in a cool, dry place, away from direct sunlight and heat sources.
- Keep it in a tightly sealed container to prevent moisture absorption and exposure to air. Moisture can hydrolyze the compound, reducing its effectiveness and potentially generating hazardous by - products.
- Store it separately from incompatible substances, such as strong acids, bases, and oxidizing agents, to avoid dangerous reactions.