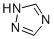Moneide Chemicals
दूरभाष: 86-315-8309571
व्हाट्सएप/वीचैट/मोबाइल: 0086-15633399667
Skype: janet-honest
Mail: sales@moneidechem.com
Address: 2-7-523 Jidong Building Materials Tangshan, Hebei 064000 China
|
Chemical Name |
Bromophemol blue |
|
CAS No. |
115-39-9 |
|
Molecular formula |
C19H10Br4O5S |
|
EINECS No. |
204-086-2 |
|
Molecular weight |
669.96 |
|
Molecular Structure |
|
|
Details |
Appearance: Almost yellow or light red powder Ph change range: 3.0(yellow)~4.6(blue purple) Solubility in alcohol: Passes test Sulfated ash: 0.25%max. Absorptivity (L/cm·g): 115min. Solubility: solve in alcohol or lye, decline solve in water and aether. Packing: 25kg/ fibre drum |
|
Main Application |
Acid-base indicator, pH 3.0(yellow)~4.6 (purple) |
What is bromophenol blue used for?
Bromophenol blue is a pH indicator and dye commonly used in laboratory settings. Its primary application is as a visual pH indicator, changing from yellow (acidic conditions) to blue (basic conditions) around pH 3.0–4.6. It is frequently added to loading dyes in gel electrophoresis to monitor the progress of DNA or protein migration. Additionally, it serves as a tracking dye, helping researchers estimate how far samples have traveled in agarose or polyacrylamide gels. Beyond electrophoresis, bromophenol blue is sometimes used in titrations and microbiology assays to detect pH changes in reactions.
Why does bromophenol blue turn yellow?
Bromophenol blue turns yellow in acidic conditions (pH below ~3.0) due to a structural change in its molecular form. The dye belongs to the sulfonphthalein family, and its color shift results from protonation (gaining hydrogen ions) in acidic environments, altering its light absorption properties. At neutral or alkaline pH, it appears blue because of deprotonation. This reversible color change makes it a useful pH indicator in experiments where visual monitoring of acidity or alkalinity is required, such as in titrations or buffer preparation.
What is the role of bromophenol blue in loading dye?
In loading dye for gel electrophoresis, bromophenol blue serves two key roles. First, it acts as a tracking dye, allowing researchers to monitor the migration of DNA or protein samples through the gel. Second, because it is a small, negatively charged molecule, it moves ahead of most biomolecules, providing a visual reference for when to stop electrophoresis before samples run off the gel. The dye also helps ensure even sample loading by increasing the density of the solution, preventing diffusion in the well. Its distinct color change at different pH levels can additionally indicate buffer conditions during the run.