|
Kemia Nomo
|
1, 10-Phenanthroline monohydrate
|
|
Sinonimoj
|
o-Phenanthroline monohydrate; 1,10-Phenanthroline hydrate
|
|
Molekula formulo
|
C12H10N2O
|
|
CAS No.
|
5144-89-8
|
|
EINECS No.
|
200-629-2
|
|
Molekula pezo
|
198.22
|
|
Molekula Strukturo
|
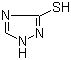
|
|
Detaloj
|
Appearance:White powder
Assay: 99% min.(AR)
Iron complex compound molar absorption coefficient(L/ cm. Mol): 1.15 X 104 min.
Ignition residue(Sulfate): 0.1% max.
Ethanol dissolved test:Qualified
Moisture: 8.5-10.0%
Packing: 1kg/bottle, 25kg/fibre drum
|
|
Ĉefa Apliko
|
Used as redox indicator, reagents for determining ferrous.
|
1,10 - Phenanthroline Monohydrate is a remarkable chemical compound that finds extensive use across diverse industries and scientific research fields. Our company takes pride in offering top - tier 1,10 - Phenanthroline Monohydrate, ensuring it meets the highest quality and purity standards for our global clientele.
Chemical Properties
1,10 - Phenanthroline Monohydrate has the chemical formula
. It appears as a white to light - yellow crystalline powder. This compound has a melting point typically around 93 - 94 °C. It is sparingly soluble in water but shows good solubility in many organic solvents like ethanol and methanol. Its chemical structure, with two nitrogen atoms in a heterocyclic aromatic ring, endows it with unique chelating properties, which are crucial for its various applications.
Diverse Applications
In Analytical Chemistry
1,10 - Phenanthroline Monohydrate is a highly valuable reagent in analytical chemistry. It forms stable, colored complexes with many metal ions, most notably iron(II). This property makes it an essential tool for the quantitative determination of metal ions in samples. In environmental analysis, it can be used to detect trace amounts of metals in water sources, helping to monitor water quality. In the mining industry, it aids in the analysis of metal ores, enabling accurate assessment of metal content.
In Coordination Chemistry and Catalysis
In coordination chemistry, 1,10 - Phenanthroline Monohydrate is widely used as a ligand. It can coordinate with metal ions to form coordination complexes, which often exhibit unique chemical and physical properties. These complexes are not only of fundamental research interest but also find applications in catalysis. For example, some iron - 1,10 - phenanthroline complexes can catalyze certain organic reactions, such as oxidation reactions, with high efficiency and selectivity.
In Biological and Medicinal Research
Recent studies have shown that 1,10 - Phenanthroline Monohydrate and its metal complexes may have potential applications in biological and medicinal research. Some metal - phenanthroline complexes have been investigated for their antibacterial, antifungal, and even anticancer properties. Although still in the research stage, these findings open up new possibilities for the development of novel drugs and therapeutic agents.
Uncompromising Quality Control
Our production of 1,10 - Phenanthroline Monohydrate follows strict quality control procedures. We utilize state - of - the - art manufacturing facilities and advanced purification techniques to ensure the highest purity of the product. Each batch undergoes rigorous testing in our in - house, well - equipped laboratories. We analyze the product for its chemical composition, purity, and physical properties. By maintaining strict quality standards, we guarantee that our customers receive a consistent and reliable product that meets or exceeds industry requirements.
Flexible Packaging and Efficient Delivery
We understand that different customers have different packaging needs. Therefore, we offer 1,10 - Phenanthroline Monohydrate in a variety of packaging options. Whether you need small sample vials for research purposes or large - scale industrial containers, we can accommodate your requirements. Our efficient delivery system ensures that your order is shipped promptly and arrives at your location in perfect condition, no matter where you are in the world. We keep you informed about the shipping status of your order throughout the process.
If you are in search of a reliable supplier of high - quality 1,10 - Phenanthroline Monohydrate for your research, industrial, or manufacturing needs, look no further. Contact us today to learn more about our product, get a quote, and discover how we can support your projects.
What is 1,10-phenanthroline monohydrate used for?
1,10-Phenanthroline monohydrate is primarily employed as a chelating agent and analytical reagent in chemistry. It forms stable, colored complexes with transition metals, particularly iron(II), making it valuable for spectrophotometric determination of iron concentrations in water and biological samples. The compound also serves as a redox indicator in titrations due to its distinct color change upon metal binding. Additionally, it finds use in organic synthesis, corrosion inhibition studies, and as a catalyst in certain chemical reactions. Its ability to selectively bind metal ions makes it a versatile tool in both industrial and laboratory settings.
What is the feature of 1,10-phenanthroline monohydrate?
The key feature of 1,10-phenanthroline monohydrate is its strong chelating ability, especially for iron(II), forming a bright red-orange complex detectable at 510 nm. This property enables highly sensitive and selective iron quantification in analytical chemistry. The monohydrate form ensures stability and solubility in aqueous solutions. Its planar, aromatic structure facilitates π-π interactions, enhancing its utility in supramolecular chemistry. The compound also exhibits fluorescence quenching when bound to metals, a feature exploited in sensor development. These characteristics make it indispensable for precise metal ion analysis and coordination chemistry studies.
Is 1,10-phenanthroline monohydrate toxic?
Yes, 1,10-phenanthroline monohydrate is moderately toxic and requires careful handling. It can cause irritation to the skin, eyes, and respiratory tract upon contact or inhalation. Ingestion may lead to gastrointestinal distress and potential systemic toxicity. While not classified as a severe carcinogen, prolonged exposure should be avoided due to possible chronic health effects. Safety measures like gloves, goggles, and fume hoods are recommended during use. Proper disposal is essential to prevent environmental contamination, as its metal-chelating properties can disrupt aquatic ecosystems. Always consult Safety Data Sheets (SDS) for specific handling protocols.






























