Moneide Chemicals
Tel: 86-315-8309571
WhatsApp/WeChat/Mobile: 0086-15633399667
Skype: janet-honest
Mail: sales@moneidechem.com
Adresse: 2-7-523 Jidong Baumaterialien Tangshan, Hebei 064000 China
Bromocresol Green
|
Chemischer Name |
Bromocresol green |
|
Synonyme |
3',3'',5',5''-Tetrabromo-m-cresolsulfonephthalein; BCG |
|
Summenformel |
C21H14Br4O5S |
|
CAS-Nr. |
76-60-8 |
|
EINECS-Nr. |
200-972-8 |
|
Molekulargewicht |
698.02 |
|
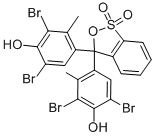
Molekulare Struktur |
|
|
Details |
Appearance: White or pale grey crystal PH change range: 3.8(yellow green)-5.4(blue) Maximal absorption wavelength (nm): λ1 (PH3.8) 440-445 λ2 (PH5.4) 615-618 Quality absorpting coefficient (L/cm·g): α1 [λ1(PH3.8,dry sample)] 24-28 α2 [λ2(PH5.4,dry sample)] 53-58 Solubility in alcohol: Passes test Ignition residue (sulfate): 0.25%max Loss on drying: 3.0%max Solubility: easy solve in alcohol, aether and benzene, decline solve in water. Packing: 25kg/fibre drum |
|
Hauptanwendung |
Used as acid and alkali indicators, cell dye. |
- pH Determination in Solutions: Bromocresol Green is widely used in laboratories for the determination of pH in aqueous and non - aqueous solutions. It can be added to samples as an indicator in titration experiments, where the color change indicates the endpoint of the reaction. For example, in the titration of weak acids with strong bases, the color change of Bromocresol Green provides an accurate visual indication of the equivalence point, allowing for precise determination of the acid concentration.
- Buffer Solution Preparation: It is also used in the preparation of buffer solutions. By carefully adjusting the pH of a solution containing Bromocresol Green, buffer solutions with specific pH values can be prepared. These buffer solutions are crucial in many chemical and biological experiments, as they help maintain a stable pH environment, which is essential for the proper functioning of enzymes, proteins, and other biomolecules.
- Cell Culture and Biological Assays: In biological research, Bromocresol Green can be used in cell culture media to monitor the pH of the culture environment. Maintaining the correct pH is vital for the growth and survival of cells. The color change of Bromocresol Green provides a quick and easy way to assess the pH of the cell culture medium, ensuring optimal conditions for cell growth. Additionally, it can be used in certain biological assays to indicate changes in pH that occur during biochemical reactions.
- Medical Diagnosis: In some medical diagnostic tests, Bromocresol Green may be used as a component in test kits. For example, in certain urine tests, the presence of specific substances can cause a change in the pH of the urine, which can be detected using Bromocresol Green as an indicator. This can provide valuable information for the diagnosis of various medical conditions.
- Quality Control in Manufacturing Processes: In industrial settings, Bromocresol Green can be used for quality control in manufacturing processes that involve pH - sensitive reactions. For example, in the production of pharmaceuticals, food and beverages, and cosmetics, the pH of the final product needs to be carefully controlled. Bromocresol Green can be used to test the pH of samples during the production process, ensuring that the products meet the required quality standards.
- Water Treatment: It can also be used in water treatment plants to monitor the pH of water. Maintaining the correct pH in water treatment processes is essential for effective removal of contaminants and prevention of corrosion in pipes and equipment. The use of Bromocresol Green allows for easy and quick assessment of the pH of water samples, enabling operators to make necessary adjustments to the treatment process.
- Bromocresol Green is harmful if swallowed, inhaled, or absorbed through the skin.
- It may cause skin and eye irritation. Prolonged or repeated exposure may have adverse effects on health.
- Contact with strong oxidizing agents can lead to dangerous reactions.
- When handling this product, always wear appropriate personal protective equipment, including chemical - resistant gloves, safety goggles, and a lab coat or protective apron.
- Work in a well - ventilated area, preferably under a fume hood, to avoid inhalation of dust or fumes.
- In case of contact with skin, immediately wash the affected area with plenty of water for at least 15 minutes and seek medical advice. If the compound gets into the eyes, rinse the eyes thoroughly with water for at least 15 minutes, lifting the eyelids occasionally, and then seek immediate medical attention.
- Do not eat, drink, or smoke in the area where Bromocresol Green is being used.
- Store Bromocresol Green in a cool, dry place, away from direct sunlight and heat sources.
- Keep it in a tightly sealed container to prevent moisture absorption and contact with air, which could potentially affect its chemical properties.
- Store it separately from incompatible substances such as strong oxidizing agents and acids to avoid dangerous reactions.
What does the bromocresol green indicator test for?
Bromocresol green is primarily used to detect pH changes in the moderately acidic range, serving as a visual indicator for acidity levels in chemical and biological systems. This sulfonphthalein dye undergoes a distinct color transition that makes it particularly valuable for monitoring acid production in microbial culture media. Clinical laboratories employ it to measure serum albumin levels through a specialized colorimetric assay. The indicator also finds application in titration experiments where a clear visual endpoint is required between pH 3.8 and 5.4. Its reliable performance in aqueous solutions has established it as a standard tool for monitoring biochemical reactions that generate or consume hydrogen ions within this specific pH window.
At what pH does bromocresol green change color?
Bromocresol green exhibits its characteristic color transition across the pH range of 3.8 to 5.4, changing from yellow in acidic conditions to blue in basic solutions. The midpoint of this transition occurs around pH 4.7, where the indicator appears green - an equal mixture of both colored forms. This relatively narrow transition range makes it especially sensitive to small pH variations in weakly acidic environments. The color change results from structural alterations in the dye molecule as it gains or loses protons, modifying its light absorption properties. The sharpness of this transition allows for precise visual determination of pH endpoints in analytical procedures where other indicators might show gradual changes.
What is the bromocresol green method used for?
The bromocresol green method serves as a widely adopted technique for quantifying serum albumin concentrations in clinical diagnostics. This specific application exploits the dye's selective binding to albumin proteins at pH 4.2, forming a colored complex measurable by spectrophotometry. Environmental scientists utilize modified versions of this method to determine aluminum concentrations in water samples. In biochemistry, it helps monitor enzyme activities that produce pH changes within its sensitive range. The method's reliability stems from the indicator's stable color complex formation and excellent reproducibility under controlled conditions. Its adaptability to automated analyzers has made it a mainstay in medical laboratories for routine albumin testing, providing rapid results with good correlation to more elaborate measurement techniques.