|
Chemical Name
|
Phenolphthalein
|
|
Synonyms
|
3,3-Bis(4-hydroxyphenyl)phthalide;
3,3-BIS(4-HYDROXYPHENYL)-1(3H)-ISOBENZOFURANONE
|
|
Molecular formula
|
C20H14O4
|
|
CAS No.
|
77-09-8
|
|
EINECS No.
|
201-004-7
|
|
Molecular weight
|
318.33
|
|
Molecular Structure
|
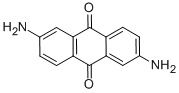
|
|
Details
|
Appearance: white or light yellow tiny triclinic crystal
Visual transition interval (PH): 8.0(Colorless)-10.0(Reddish purple)
Mass absorption coefficient: 68.0---75.0(L/cm·g)
Solubility in Ethanol: Pass
Loss on drying: 1.0 % max
Residue on ignition (sulfate): 0.05%max
Packing: 25kg/ fibre drum
|
|
Main Application
|
Used as HP indicator to check the deposit, cadmium, magnesium.
|
Applications
Phenolphthalein stands as a pivotal chemical compound, playing an integral role across a wide spectrum of scientific, industrial, and educational arenas. Our company is committed to delivering premium - grade Phenolphthalein, ensuring it adheres to the most exacting standards of quality and purity, catering to customers globally.
Chemical Attributes
Phenolphthalein has the chemical formula
. It typically presents as a white to off - white crystalline solid. This compound is sparingly soluble in water but dissolves readily in ethanol and other organic solvents. Phenolphthalein exhibits a characteristic melting point in the range of 258 - 262 °C. Its unique chemical structure confers it with properties that are indispensable in multiple applications.
Extensive Applications
Acid - Base Titrations
Phenolphthalein is perhaps best - known for its role as an indicator in acid - base titrations. In an alkaline solution, it turns a vibrant pink color, while in an acidic medium, it remains colorless. This distinct color change makes it an invaluable tool for accurately pinpointing the endpoint of titration reactions. In laboratories engaged in pharmaceutical development, environmental monitoring, and food and beverage quality assessment, Phenolphthalein is relied upon to conduct precise chemical analyses, guaranteeing the accuracy of results.
pH Detection
Owing to its high sensitivity to pH variations, Phenolphthalein is widely used in pH - detection applications. It can be incorporated into pH - test papers or sensors, enabling rapid and straightforward determination of the pH value of a solution. This is especially crucial in industries where maintaining the correct pH level is of utmost importance, such as in the production of personal care products, where the pH of formulations impacts product stability and skin - friendliness.
Educational Purposes
In educational institutions, Phenolphthalein serves as an outstanding teaching aid. Its vivid color transformation during acid - base reactions makes it an engaging visual tool for students learning about chemical reactions and the concept of pH. Teachers frequently utilize Phenolphthalein in classroom experiments to captivate students and facilitate a deeper understanding of fundamental chemical principles.
Stringent Quality Assurance
Our production of Phenolphthalein is governed by the strictest quality control protocols. We employ advanced manufacturing technologies and cutting - edge equipment in our production facilities. Every batch of Phenolphthalein is subjected to comprehensive testing in our in - house, ISO - accredited laboratories. We meticulously analyze the product for its chemical purity, physical characteristics, and performance in relevant applications. By ensuring that our Phenolphthalein not only meets but surpasses international quality benchmarks, we offer our customers a dependable and consistent product.
Custom Packaging and Expedited Delivery
We recognize that customers have diverse packaging requirements. Hence, we provide Phenolphthalein in a range of packaging options. Whether you require small - scale quantities in vials for laboratory research or large - volume industrial - sized containers, we can fulfill your needs. Our efficient logistics network ensures that your order is dispatched promptly and arrives in pristine condition, regardless of your geographical location. We are dedicated to minimizing delivery times and keeping you informed about the progress of your order throughout the shipping process.
If you are seeking a trustworthy supplier of high - quality Phenolphthalein for your research, industrial, or educational endeavors, our company is your optimal choice. Contact us today to explore our product range, obtain pricing details, and discover how we can support your specific projects.
What is phenolphthalein and why is it used?
Phenolphthalein is a synthetic pH indicator widely used in chemistry to detect alkaline conditions. It transitions from colorless (in acidic or neutral solutions) to bright pink/fuchsia (in basic solutions) around pH 8.2–10.0, making it ideal for acid-base titrations, particularly with strong bases like NaOH. Beyond titrations, it serves in educational labs to demonstrate pH changes and in forensic investigations as a presumptive test for blood due to its reaction with hemoglobin. Its clear, vivid color change provides a reliable visual endpoint, ensuring accuracy in quantitative chemical analysis.
What does phenolphthalein turn pink in?
Phenolphthalein turns pink in alkaline (basic) solutions with a pH above ~8.2. This color change occurs when the molecule loses hydrogen ions (deprotonation) in high-pH environments, altering its structure to absorb green light and reflect pink. The intensity deepens as pH rises, becoming brightest around pH 10. Common applications include testing the alkalinity of soaps, detergents, and water samples. Notably, in very strong bases (pH >13), it turns colorless again, which is why it's primarily used for moderate alkalinity detection.
Why is phenolphthalein hazardous?
Phenolphthalein is classified as hazardous due to its potential carcinogenicity and toxicity. Long-term exposure or ingestion may increase cancer risk, as studies linked it to tumor growth in rodents. It can also cause skin/eye irritation and allergic reactions upon contact. Once used in laxatives, it was banned in many countries for medicinal use due to safety concerns. In labs, proper PPE (gloves, goggles) is required when handling it. While useful as an indicator, its chemical hazards warrant careful disposal and restricted use in educational and industrial settings.






























