|
Chemiese naam
|
1,10-Phenanthroline hydrochloride
|
|
Sinonieme
|
1,10-Phenanthroline monohydrochloride
|
|
Molekulêre formule
|
C12H8N2 ·HCl ·H2O
|
|
CAS No.
|
3829-86-5
|
|
EINECS No.
|
223-325-1
|
|
Molekulêre gewig
|
234.69
|
|
Molekulêre struktuur
|
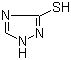
|
|
Besonderhede
|
Appearance: Colorless or white crystalline powder
Loss on drying: 7.8% max
Sulfated ash: 0.05% max
Iron complex Mole absorption coefficient:1.15×104
Solubility in water: Passes test
Solubility: Solve in water and alcohol
Packing: 25kg/ fibre drum
|
|
Hooftoepassing
|
Aanwyser
|
1,10 - Phenanthroline Hydrochloride is a chemical compound of great significance, finding wide - spread use in various scientific and industrial fields. Our company is committed to providing top - tier 1,10 - Phenanthroline Hydrochloride, guaranteeing it meets the most stringent quality and purity standards for our global clientele.
Chemical Characteristics
1,10 - Phenanthroline Hydrochloride has the chemical formula
. It typically appears as a white to off - white crystalline powder. This compound is highly soluble in water, which is a crucial property for its applications. Solubility in water allows for easy incorporation into aqueous solutions used in different processes. Its structure, based on the 1,10 - phenanthroline framework with an added hydrochloride group, endows it with unique chemical reactivity. The presence of the hydrochloride group can influence its coordination behavior with metal ions and its interactions in various chemical environments.
Multifaceted Applications
In Analytical Chemistry
In the realm of analytical chemistry, 1,10 - Phenanthroline Hydrochloride serves as an essential reagent. It forms stable complexes with a variety of metal ions, especially iron(II). This property is exploited for the quantitative determination of metal ions in samples. For instance, in environmental analysis, it can be used to detect trace amounts of iron and other metals in water samples. By forming colored complexes, the concentration of these metals can be accurately measured using spectroscopic techniques. In the analysis of metal ores, it helps in precisely determining the metal content, which is vital for the mining and metallurgical industries.
In Coordination Chemistry
As a ligand in coordination chemistry, 1,10 - Phenanthroline Hydrochloride plays a pivotal role. It coordinates with metal ions to form coordination complexes. These complexes often exhibit unique chemical and physical properties. Some of these complexes are used as catalysts in chemical reactions. For example, certain metal - 1,10 - phenanthroline hydrochloride complexes can catalyze organic reactions such as hydrogenation and oxidation reactions. The specific structure of the ligand - metal complex can be tailored to enhance the selectivity and efficiency of these reactions.
In Biological and Medicinal Research
Recent research has explored the potential applications of 1,10 - Phenanthroline Hydrochloride in biological and medicinal fields. Some metal - 1,10 - phenanthroline hydrochloride complexes have shown antibacterial and antifungal properties. Additionally, they may have potential in cancer research, with studies suggesting that certain complexes can interact with DNA and disrupt the growth of cancer cells. Although still in the research and development stage, these findings open up new avenues for the development of novel drugs and therapeutic agents.
Rigorous Quality Control
Our production of 1,10 - Phenanthroline Hydrochloride adheres to the strictest quality control procedures. We utilize state - of - the - art manufacturing facilities and advanced purification techniques. Each batch of the product undergoes comprehensive testing in our in - house, well - equipped laboratories. We analyze its chemical purity, ensuring that it is free from impurities that could affect its performance. The physical properties, such as solubility and crystal structure, are also carefully examined. By maintaining these high - level quality standards, we offer our customers a consistent and reliable product that performs optimally in their applications.
Flexible Packaging and Swift Delivery
We recognize that different customers have diverse packaging requirements. Therefore, we offer 1,10 - Phenanthroline Hydrochloride in a wide range of packaging options. Whether you need small sample vials for research purposes or large - scale industrial containers, we can accommodate your needs. Our efficient logistics network ensures that your order is shipped promptly and arrives at your location in perfect condition, regardless of where you are in the world. We keep you informed about the shipping status of your order at every stage, providing you with peace of mind.
If you are in search of a reliable supplier of high - quality 1,10 - Phenanthroline Hydrochloride for your research, industrial, or manufacturing needs, look no further. Contact us today to learn more about our product, obtain a quote, and discover how we can support your projects.
What is 1,10-phenanthroline Hydrochloride used for?
1,10-Phenanthroline hydrochloride is primarily used as a water-soluble derivative of 1,10-phenanthroline, offering enhanced solubility for applications in acidic or aqueous systems. It serves as a highly effective chelating agent for transition metals, particularly iron(II), enabling sensitive spectrophotometric detection in environmental and biological samples. The compound is also employed in electrochemical studies as a redox mediator, in organic synthesis as a catalyst, and in corrosion inhibition research. Its improved solubility compared to the neutral ligand makes it valuable for analytical procedures requiring precise metal ion quantification in varied pH conditions.
What is the purpose of using 1,10-phenanthroline Hydrochloride?
The purpose of using 1,10-phenanthroline hydrochloride is to overcome solubility limitations of the neutral ligand in aqueous or acidic environments. Its hydrochloride form ensures consistent reactivity and stability, making it ideal for iron(II) detection in complex matrices like biological fluids or industrial wastewater. The compound’s ability to form stable, colored complexes with metals allows for accurate spectrophotometric analysis. Additionally, it functions as a versatile tool in coordination chemistry, catalysis, and materials science, where controlled metal-chelate interactions are critical for experimental success.
What is the advantage of 1,10-phenanthroline?
The primary advantage of 1,10-phenanthroline is its exceptional selectivity and sensitivity toward iron(II), enabling detection at trace levels with minimal interference. Its rigid, planar structure forms highly stable metal complexes, ensuring reliable analytical results in spectrophotometry. The ligand’s redox activity allows dual functionality as both an indicator and catalyst. Derivatives like the hydrochloride and monohydrate forms further enhance versatility by offering tailored solubility for specific applications. These properties make it indispensable in environmental monitoring, industrial quality control, and biochemical research, where precision and adaptability are paramount






























